
05 Dec, 2018 Alnylam R&D Day 2018
On December 6th, 2018, we will be hosting an R&D Day in New York City where Alnylam management and key opinion leaders will discuss our late stage clinical efforts, in addition to next wave programs and platform advances. Scroll down to see the R&D Day agenda and links to the presentations. Learn more about our science and the science of RNAi. Click here to see our pipeline of RNAi therapeutics.
To view the webcast, click here. A replay of the webcast will be available at that link on December 7th.
To view the R&D Day Presentation, click here.
Speakers:
 John Maraganore, Ph.D. – Chief Executive Officer
John Maraganore, Ph.D. – Chief Executive Officer
Since 2002, Dr. John Maraganore has served as the CEO and a Director of Alnylam.
Prior to Alnylam, Dr. Maraganore served as an officer and a member of the management team for Millennium Pharmaceuticals, Inc. As Senior Vice President, Strategic Product Development for Millennium, he was responsible for the company’s product franchises in oncology, and cardiovascular, inflammatory, and metabolic diseases. He was previously Vice President, Strategic Planning and M&A and, prior to that, he was General Manager of Millennium BioTherapeutics, Inc., a former subsidiary of Millennium. Before Millennium he served as Director of Molecular Biology and Director of Market and Business Development at Biogen, Inc. At Biogen, Dr. Maraganore invented and led the discovery and development of ANGIOMAX® (bivalirudin) for injection, formerly HIRULOGTM and currently marketed by The Medicines Company. Prior to Biogen, Dr. Maraganore was a scientist at ZymoGenetics, Inc., and the Upjohn Company.
Dr. Maraganore received his Masters of Science and Ph.D. in biochemistry and molecular biology at the University of Chicago. He is the Chair of the Board for Agios Pharmaceuticals. Dr. Maraganore is Chair of the Biotechnology Industry Organization (BIO) Board, and is a member of the BIO Executive Committee.
 Akshay Vaishnaw, M.D., Ph.D. – President, Research & Development
Akshay Vaishnaw, M.D., Ph.D. – President, Research & Development
Dr. Akshay Vaishnaw joined Alnylam in 2006, coming from Biogen, Inc., where he was most recently Senior Director, Translational Medicine.
In his 7 years at Biogen, Dr. Vaishnaw was involved in many aspects of clinical research and business development, and led the effort for the approval of AMEVIVE® (alefacept) for psoriasis.
Dr. Vaishnaw received his M.D. from the University of Wales College of Medicine, UK, and his Ph.D. from the University of London, UK, in Molecular Immunology. He is a Fellow of the Royal College of Physicians, UK.In addition, he has published papers in leading scientific journals and authored several textbook chapters relating to autoimmune disease. Dr. Vaishnaw is a member of the Scientific Advisory Board of Scholar Rock, and a member of the Board of Directors for Visterra, Inc., and for Editas Medicine.
 Eric Green, Vice President & General Manager, TTR Program
Eric Green, Vice President & General Manager, TTR Program
Eric Green joined Alnylam in 2015 and is currently Vice President and General Manager of the TTR program. Previously, Eric worked at Synageva BioPharma, where he was Vice President of Program and Alliance Management. In that role he was responsible for the oversight, management, and leadership of the company’s lead compound being developed as an enzyme replacement therapy for an ultra-orphan genetic disease. Prior to Synageva, he worked as Senior Director, Product Development and Program Management at Infinity Pharmaceuticals, where he developed the clinical and commercial product strategy and led the operational execution for the development of a small molecule product being investigated in non-small cell lung cancer. Before working at Infinity, he spent over eight years at Genzyme Corporation, where he served in roles of increasing responsibility in program and brand management for multiple commercial oncology products.
Eric received his Bachelor of Science in Chemical Engineering from the University of Michigan, a Masters in Chemical Engineering from the Massachusetts Institute of Technology (MIT), and an MBA from the MIT Sloan School of Management.
 Puskal Garg, M.D. – Chief Medical Officer
Puskal Garg, M.D. – Chief Medical Officer
Dr. Pushkal Garg joined Alnylam in 2014 with 15 years of experience in clinical drug development. He oversees clinical research & operations, biometrics & data management, and medical writing groups.
Prior to joining Alnylam, Dr. Garg served as Vice President, Global Clinical Research, Immunoscience at Bristol-Myers Squibb (BMS). He was the strategic leader of the Immunoscience franchise and successful developer of multiple clinical assets across the areas of rheumatology, gastroenterology, nephrology, and transplantation. While at BMS, he was instrumental to the late-stage development and approval of Nulojix® (belatacept) for kidney transplant recipients, and for supplementary biologics license applications (BLAs) for Orencia® (abatacept). Preceding this, Dr. Garg held various roles at Millenium Pharmaceuticals, overseeing the clinical development of multiple small molecule and biologic therapeutics for the treatment of inflammatory disorders.
Dr. Garg received a Bachelor of Arts with high honors in Biochemistry from the University of California, Berkeley, and his M.D. from the University of California, San Francisco. He completed residency training in Internal Medicine at UCSF, was a fellow in the Robert Wood Johnson Clinical Scholars Program at Johns Hopkins University, and served on the faculty of Harvard Medical School and the Brigham & Women’s Hospital in Boston prior to joining the industry. Dr. Garg is a member of the Board of Directors of SQZ Biotechnologies (SQZ).
 Andy Orth, Senior Vice President, Head of the U.S. Region
Andy Orth, Senior Vice President, Head of the U.S. Region
Andy Orth joined Alnylam in 2016 and is currently Senior Vice President, Head of U.S. Region, which includes U.S. Business Account Teams, Market Access, Marketing, Patient Services and Operations. Previously, Andy served as Alnylam’s Vice President of Commercial Practice where he helped to build out Alnylam’s commercial organization and infrastructure as the company readied itself for the launch of ONPATTRO (patisiran).
Before joining Alnylam, Andy spent six years with Biogen, during which he helped launch multiple products into the US Market, with his last position being Vice-President Global Commercial Strategy, Head of Decision Support.Earlier, he spent time in Finance, Analytics and Consulting roles with Genzyme, Russell Reynolds and Amgen.
Andy received his MBA from The Johnson Graduate School of Management at Cornell University and his undergraduate degree at The University of Wisconsin.
 John D. Phillips, Ph.D.
John D. Phillips, Ph.D.
Dr. John D. Phillips is a Professor in the Department of Medicine, Hematology Division and administratively, the Director of the Health Sciences Center Core facilities at the University of Utah. He is also the Director of the Cell Therapy and Regenerative Medicine Facility at the University of Utah School of Medicine.
Dr. Phillips has been working in the heme biosynthesis field for the past 23 years focusing on the porphyrias, rare diseases in which one of eight steps in heme biosynthesis is defective. During this time, he has been defining transcriptional, translational and post-translational mechanisms that regulate heme biosynthesis while working with Dr. James Kushner. He has a long history of productive collaboration with researchers in this field and has been funded continuously for his work in the porphyria field.In 2010 he took responsibility as the principle investigator of Utah site for the Office of Rare Disease and NIDDK funded Porphyria Consortium, when Jim Kushner retired.
Dr. Phillips is also the principle investigator of the Center for Iron and Heme Disorders, an NIDDK funded program to expand the support for researchers nationally working in the erythropoiesis field. He is an active participant in the Porphyria Consortium and works closely with the American Porphyria Foundation engaging in research studies on the different porphyrias as well as training the next generation of porphyria experts.
Dr. Phillips received his Ph.D. from the Dartmouth Medical School and his Bachelor of Science in Animal Science from the University of New Hampshire. He completed his postdoctoral fellowship at the University of Utah.
 Akin Akinc, Ph.D. – Vice President & General Manager, Givosiran Program
Akin Akinc, Ph.D. – Vice President & General Manager, Givosiran Program
Dr. Akin Akinc joined Alnylam in 2003 and is currently Vice President and General Manager of the givosiran program. Prior to taking the GM role on givosiran, Akin was General Manager of the fitusiran program, which he led until the Alnylam-Sanofi alliance restructuring in January 2018. Previously, Akin served in roles of increasing responsibility and leadership in the research organization.
During his time at Alnylam he has led formulation teams and co-led interdisciplinary efforts focused on delivery of RNAi therapeutics and the development of RNAi platform technology.
Dr. Akinc received his Bachelor of Science in Chemical Engineering from Princeton University and his Ph.D. in Chemical Engineering from the Massachusetts Institute of Technology.
 Yvonne Greenstreet, MBChB – Chief Operating Officer
Yvonne Greenstreet, MBChB – Chief Operating Officer
Dr. Yvonne Greenstreet possesses 25 years of global experience in the pharmaceutical industry, where she has been in senior roles in research and development, strategy, and portfolio management. She has successfully led product development and commercialization teams in a wide range of therapy areas, bringing new medicines to patients.
Yvonne was previously Senior Vice President and Head of Medicines Development at Pfizer, and a member of the executive team for the specialty business, with accountability for a portfolio that included the immuno-inflammation, vaccine, specialty neuroscience, and rare disease areas. Prior to Pfizer, Yvonne held roles of increasing responsibility at GlaxoSmithKline, including Senior Vice President and Chief of Strategy for Research and Development, Senior Vice President for Medicines Development and Chief Medical Officer for Europe.
Dr. Greenstreet received her medical degree (MBChB) from the University of Leeds, UK, and an MBA degree from INSEAD, France. She serves as a member of the board of directors of Pacira Pharmaceuticals, Inc., a specialty pharmaceutical company, and Indivior PLC, a global specialty pharmaceutical business. Dr. Greenstreet also serves on the Scientific Advisory Committee of the Bill and Melinda Gates Foundation.
 Sally-Anne Hulton, M.D.
Sally-Anne Hulton, M.D.
Dr. Sally-Anne Hulton was appointed Consultant Paediatric Nephrologist at Birmingham Children’s Hospital and Honorary Senior Lecturer at University of Birmingham UK in 1995. In this position she has served as lead physician for both combined liver kidney transplantation and renal metabolic disease. She has particular expertise in the primary hyperoxalurias and established the national PH RaDaR registry and served as Chair of the OxalEurope Steering Committee (2010-2017).
Dr. Hulton is currently President of the British Association for Paediatric Nephrology and Vice President of the Renal Association. She has also served as expert member and Vice Chair of the hospital Ethics Committee (2007-2018) and Specialty Training Advisor and Chair of College Specialist Advisory Committee for Paediatric Nephrology for the Royal College of Paediatrics and Child Health (RCPCH) from 2004 -2015.
Dr. Hulton received her undergraduate degree from the University of the Witwatersrand, South Africa. Her doctoral thesis was awarded from the University of Birmingham following research at the Institute of Child Health in London. She received her fellowship in Paediatrics from the College of Physicians of South Africa (FCP SA) and the Royal College of Paediatrics and Child Health in London (FRCPCH). Dr. Hulton is also a fellow of the Royal College of Physicians (FRCP).
 Pritesh Gandhi, PharmD. – Vice President & General Manager, Lumasiran Program
Pritesh Gandhi, PharmD. – Vice President & General Manager, Lumasiran Program
Dr. Pritesh Gandhi joined Alnylam in 2014 and is currently Vice President and General Manager of the lumasiran program. Prior to taking the GM role on lumasiran, Pritesh was Vice President of Medical Affairs. Previously, he served as Associate Vice President of Global Medical Affairs at Sanofi Oncology, where he spearheaded and executed on the global publication, medical education, and medical communication strategy.
Earlier, he worked as Regional Director of Medical Scientific Relations at Alexion Pharmaceuticals, where he managed efforts to bridge the gap between the science of eculizumab and the practice of medicine in the area of paroxysmal nocturnal hemoglobinuria (PNH). Preceding Alexion, he was at Millennium Pharmaceuticals, where he was the operational lead for the EVENT registry.
Before joining the pharmaceutical industry, Pritesh held an academic appointment at Massachusetts College of Pharmacy as well as an adjunct position at The University of Massachusetts Medical School. At that time, he also practiced as Cardiovascular Pharmacotherapy Specialist at UMass Memorial Health Care.
Dr. Gandhi received his Bachelor of Science in Pharmacy and his PharmD. from the Massachusetts College of Pharmacy and Health Sciences. He completed a clinical residency at The University of Illinois Chicago, and is a Registered Pharmacist in the State of Illinois and the Commonwealth of Massachusetts.
 Kevin Fitzgerald, Ph.D. – Senior Vice President, Head of Research and Translational Science
Kevin Fitzgerald, Ph.D. – Senior Vice President, Head of Research and Translational Science
Dr. Kevin Fitzgerald joined Alnylam in 2005 and is currently Senior Vice President, Head of Research and Translational Science at Alnylam Pharmaceuticals. He has served in roles of increasing responsibility and leadership during his time at the company.
Dr. Fitzgerald’s achievements at Alnylam include leadership of the company’s RNAi delivery efforts, which resulted in two clinically validated modes of siRNA delivery, and the development of Alnylam’s clinical pipeline.
Kevin is an inventor on over 50 patents including the majority of Alnylam’s pipeline programs, and the author of over 40 papers including many in prestigious journal’s such as Nature, Cell, and The New England Journal of Medicine (NEJM). He is a co-author on the initial patisiran NEJM paper and has led multiple programs —including the inclisiran program —from discovery through pre-clinical development, regulatory submissions, and early clinical development.
Dr. Fitzgerald received his Bachelor of Science in genetics from Cornell University and his doctorate in molecular biology from Princeton University. He completed his postdoctoral fellowship in oncology at Harvard Medical School.
 Barry Greene, President
Barry Greene, President
Barry Greene joined Alnylam in 2003 and has more than 25 years of experience in healthcare, pharmaceutical, and biotechnology industries.
Prior to Alnylam, Barry was General Manager of Oncology at Millennium Pharmaceuticals, Inc., where he led the company’s global strategy and execution for its oncology business, including strategic business direction and execution, culminating in the successful approval and launch of VELCADE® (bortezomib) in mid-2003. Prior to joining Millennium in February 2001, Barry served as Executive Vice President and Chief Business Officer for Mediconsult.com. Preceding that, Barry’s past experiences include Vice President of Marketing and Customer Services for AstraZeneca, formerly AstraMerck; Vice President Strategic Integration with responsibility for the AstraZeneca North American post-merger integration; and Partner, Andersen Consulting, responsible for the pharmaceutical/biotechnology marketing and sales practice.
Barry received his Bachelor of Science degree in Industrial Engineering from University of Pittsburgh and served as Senior Scholar at Duke University, Fuqua School of Business. Barry also serves on the Boards of Acorda Therapeutics and Karyopharm Therapeutics.
 Rena Denoncourt, Senior Director, Vutrisiran Program Lead
Rena Denoncourt, Senior Director, Vutrisiran Program Lead
Rena Denoncourt joined Alnylam in 2007 and is currently the Program Leader for Vutrisiran. She has been focused on the TTR portfolio since its inception and has held positions of increasing responsibility across various expertise areas including business planning, program and alliance management, and market access. As one of Alnylam’s first commercial employees, Rena was instrumental in the development of Alnylam’s Patient Access Philosophy and the proactive Value-Based Agreement (VBA) strategy for ONPATTRO (patisiran) with US payers.
Prior to joining Alnylam, Rena earned her MBA from the MIT Sloan School of Management and a Bachelor of Science in Biology from the Massachusetts Institute of Technology.
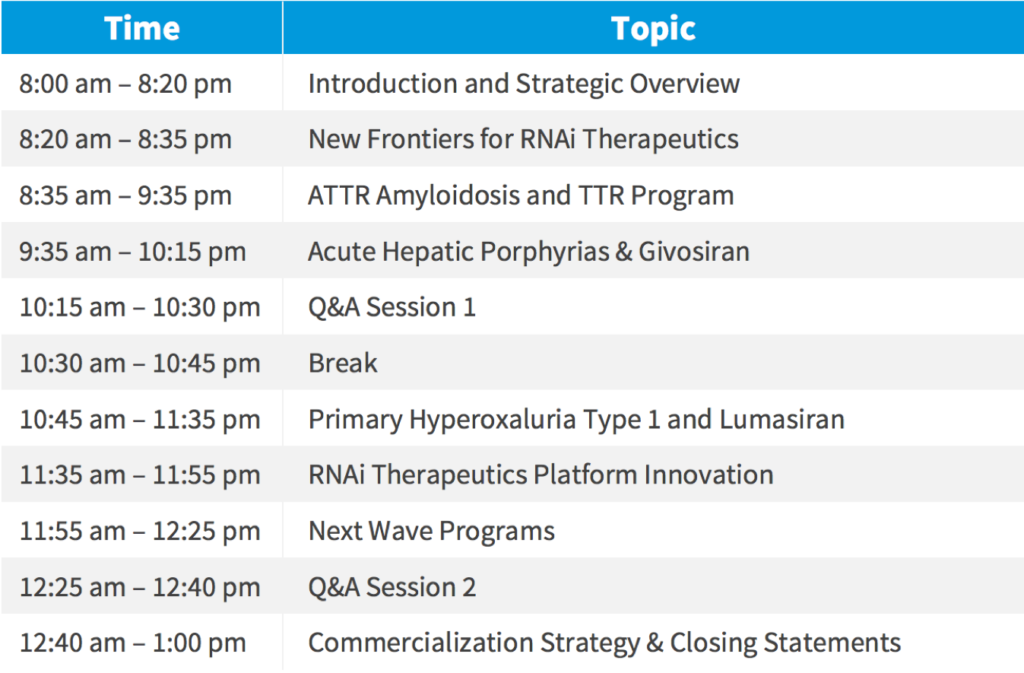
Agenda:
If you have questions, please contact:
Christine Regan Lindenboom
(Investors and Media)
617-682-4340
Josh Brodsky
(Investors)
617-551-8276


