25 Feb, 2025 Alnylam R&D Day 2025
On February 25, 2025, we hosted an R&D Day event showcasing Alnylam’s late-stage clinical efforts, next wave programs, and platform advances. The event included presentations from Alnylam senior leaders, as well as a leading expert in Huntington’s disease.
To view the webcast, click here
To view the presentation, click here
Speakers:
 Yvonne Greenstreet, M.D. – Chief Executive Officer
Yvonne Greenstreet, M.D. – Chief Executive Officer
Dr. Yvonne Greenstreet joined Alnylam in 2016 as Chief Operating Officer, was promoted to President and COO in 2020, and was appointed as a Director and Chief Executive Officer in late 2021. Yvonne has more than 25 years of experience in the Biopharmaceutical industry, driving strategy and innovation, bringing transformative medicines to patients, and building successful businesses in the US, Europe and globally.
Between 2011 and 2013, Yvonne was Senior Vice President and Head of Medicines Development at Pfizer serving on the executive team leading a rapidly growing $16bn division. Prior to Pfizer, she was at GlaxoSmithKline plc for 18 years, where she was Senior Vice President and Chief of Strategy for Research and Development. Yvonne had previously been in various positions of increasing responsibility at GSK, including Senior Vice President for Medicines Development and Chief Medical Officer for Europe.
Yvonne trained as a physician and earned her medical degree (MBChB) from The University of Leeds in the UK. She also holds an MBA from INSEAD Business school in France. She serves on the board of directors of The American Funds. Yvonne is a member of Bill and Melinda Gates Foundation Scientific Advisory Committee, the Discovery Council of Harvard Medical School, and the Council on Foreign Relations. She is also a member of the Biotechnology Industry Organization Health Section Governing Board and the board of the Biomedical Science Careers Program. Yvonne was recognized as the Healthcare Businesswomen’s Association 2024 Woman of the Year.
 Pushkal Garg, M.D. – Chief Medical Officer
Pushkal Garg, M.D. – Chief Medical Officer
Dr. Pushkal Garg joined Alnylam in 2014 with 15 years of experience in clinical drug development. He oversees all clinical development activities at Alnylam as Chief Medical Officer and Executive Vice President of Development and Medical Affairs.
Prior to joining Alnylam, Dr. Garg served as Vice President, Global Clinical Research, Immunoscience at Bristol-Myers Squibb (BMS). He was the strategic leader of the Immunoscience franchise and oversaw the development of multiple clinical assets across the areas of rheumatology, gastroenterology, nephrology, and transplantation. While at BMS, he was instrumental to the late-stage development and approval of Nulojix® (belatacept) for kidney transplant recipients, and for supplementary biologics license applications (BLAs) for Orencia® (abatacept). Preceding this, Dr. Garg held various roles at Millennium Pharmaceuticals, overseeing the clinical development of multiple small molecule and biologic therapeutics for the treatment of inflammatory disorders.
Dr. Garg received a Bachelor of Arts with high honors in Biochemistry from the University of California, Berkeley, and his M.D. from the University of California, San Francisco. He completed residency training in Internal Medicine at UCSF, was a fellow in the Robert Wood Johnson Clinical Scholars Program at Johns Hopkins University, and served on the faculty of Harvard Medical School and the Brigham & Women’s Hospital in Boston prior to joining the industry.
 John Vest, M.D. – SVP, TTR Global Development Lead
John Vest, M.D. – SVP, TTR Global Development Lead
Dr. John Vest joined Alnylam in 2015 and is currently Senior Vice President, TTR Global Development Lead. In this role Dr. Vest oversees both the clinical development strategy and clinical trial execution for Alnylam’s innovative RNAi therapeutics targeting transthyretin. He has played an instrumental role in multiple pivotal Phase 3 studies of ONPATTRO and vutrisiran. Before joining Alnylam, he worked in the Cardiovascular Translational Medicine group at Novartis where he played a key clinical role in developing the company’s early stage pipeline of compounds in the cardiovascular space. Prior to his career in industry, Dr. Vest spent over 16 years in the study and practice of medicine. Dr. Vest received his MD from Columbia University where he went on to complete a residency in Internal Medicine and fellowship in Cardiovascular Disease. He subsequently completed sub-specialty training in Clinical Cardiac Electrophysiology at the Brigham and Women’s Hospital before returning to Columbia where he joined the faculty of the Cardiology Division.
 Simon Fox, Ph.D. – VP, Program Lead, Zilebesiran
Simon Fox, Ph.D. – VP, Program Lead, Zilebesiran
Simon Fox joined Alnylam in 2021 and is currently Vice President, zilebesiran Program Lead, for which he is responsible for establishing and delivering the long-term strategy for the program. Simon has 19 years of industry experience, most of this time spent developing and commercializing innovative treatments for cardiovascular, metabolic, and hematological diseases. Prior to joining Alnylam Simon held numerous global strategic roles in both the R&D and commercial functions. He was the Global Medical Affairs Director responsible for AstraZeneca’s diabetes franchise, notably lifecycle management strategy and launching Farxiga (dapagliflozin) globally. In addition, he also held the position of Global Marketing Director for Brilinta (ticagrelor), responsible for driving strategic investment decisions for new indications. Most recently Simon was at CSL Behring as the global commercial lead for their late-stage cardiovascular program before going on to lead their hematology franchise globally which included the first approved gene therapy for hemophilia B, Hemgenix (etranacogene dezaparvovec).
Simon obtained his Bachelor of Science in chemistry, drug design and toxicology, and his Ph.D. in organic chemistry from the University of Hull, UK.
 Julia Shirvan, M.D., Ph.D. – Sr. Director, Mivelsiran Clinical Lead
Julia Shirvan, M.D., Ph.D. – Sr. Director, Mivelsiran Clinical Lead
Dr. Julia Shirvan joined Alnylam in 2024 and is currently Senior Director, Clinical Research. She is the clinical strategy lead for Mivelsiran, the most advanced CNS program with two active clinical trials. She brought 6 years of experience in clinical development, with expertise in Parkinson’s Disease (PD) genetics. She was the medical lead for multiple programs across all stages of development for PD at Biogen, where she was recognized as a CEO Top Talent. Her medical training is in neurology. She earned her M.D. from the University of Massachusetts. She completed her residency at Columbia-New York Presbyterian Hospital and her movement disorders fellowship at Mass General Brigham. During fellowship she conducted research on progression biomarkers in PD in Dr. Clemens Scherzer’s lab. Julia also earned her A.B. from Harvard College in History and Statistics and her Ph.D. in Statistics from the University of Oxford, with specialization in statistical genetics. For her doctorate, she collaborated with the National Cancer Institute to develop novel analytic methods for genome wide association studies.
 Prof. Sarah Tabrizi, M.D., Ph.D. – Joint Head of Department of Neurodegenerative Disease, UCL
Prof. Sarah Tabrizi, M.D., Ph.D. – Joint Head of Department of Neurodegenerative Disease, UCL
Prof. Tabrizi is Director of the UCL Huntington’s Disease Centre and Joint Head of Department of Neurodegenerative Disease at the UCL Queen Square Institute of Neurology. She is also a Principal Investigator at the UK Dementia Research Institute, and Honorary Consultant Neurologist at the National Hospital for Neurology and Neurosurgery.
In addition to basic bench science focused on cellular mechanisms of neurodegeneration in Huntington’s disease (HD), Prof. Tabrizi also leads a large translational research program in HD that is working towards finding effective disease-modifying treatments. She has published over 400 peer-reviewed publications to date, and serves on several expert panels including for the UK HD association. Prof. Tabrizi co-founded the UK All Party Parliamentary Group for HD in 2010, and was elected a Fellow of the UK Academy of Medical Sciences in 2014. Prof. Tabrizi has received many prizes and awards, including the 2022 MRC Millennium Medal for her outstanding achievements in medical research. In 2023 she received the Arvid Carlsson Award from Lund University for her research in HD mechanisms and therapeutics. In 2024 she was elected to the Fellowship of the Royal Society and to the U.S. National Academy of Medicine.
Prof. Tabrizi studied medicine at the University of Edinburgh in 1992 where she graduated with the Gold Medal for most distinguished medical graduate. She has worked on research into neurodegenerative diseases since her Ph.D. as an MRC clinical training fellow at UCL. After clinical training, she obtained a DH National Clinician Scientist Fellowship in 2002 to work on protein misfolding at UCL. She was promoted to Senior Lecturer and Honorary Consultant Neurologist in 2003, and to Full Professor in 2009.
 Kevin Sloan, Ph.D. – VP, Program Lead, Early CNS Programs
Kevin Sloan, Ph.D. – VP, Program Lead, Early CNS Programs
Dr. Kevin Sloan joined Alnylam in 2018 and is currently Vice President, Development Programs, and Program Lead for several programs including ALN-HTT02 for Huntington’s disease. He has a background in translational science and early-stage drug development and played a key role in the expansion of Alnylam’s pipeline of development candidates and early-stage programs over the past seven years.
Kevin has over twenty years of experience working in the biotechnology industry. Prior to joining Alnylam, Kevin held various R&D and cross-functional leadership positions at Intellia Therapeutics, Novartis Institutes for BioMedical Research, Vertex Pharmaceuticals and Biogen, with roles spanning basic research through clinical proof-of-concept studies. He has a passion for drug development and patient impact, and a breadth of experience across multiple indication areas, platform modalities & company sizes.
Kevin earned his Ph.D. at Tufts University School of Medicine, an MA at Boston University, and a BA at Macalester College. He lives in Newton, Massachusetts with his wife and two daughters.
 Kevin Fitzgerald, Ph.D. – Chief Scientific Officer
Kevin Fitzgerald, Ph.D. – Chief Scientific Officer
Dr. Kevin Fitzgerald joined Alnylam in 2005 as Associate Director of Research and has served in roles of increasing responsibility and leadership since that time. Kevin was promoted to Chief Scientific Officer of Alnylam in May of 2019; he is also Executive Vice President and Head of Research at Alnylam and has 24 years of drug discovery experience.
His achievements at Alnylam include leadership of the company’s RNAi delivery efforts, resulting in two clinically validated modes of siRNA delivery, and the development of Alnylam’s RNAi therapeutic novel class of medicines. He is an inventor on over 50 patents including the majority of Alnylam’s marketed and pipeline programs, as well as an author of over 50 papers including many in prestigious journals such as Nature, Cell, and New England Journal of Medicine. He has led multiple programs — including Alnylam’s inclisiran program — from discovery through pre-clinical development, regulatory submissions, and early clinical development. Prior to 2005, Kevin was at Bristol-Myers Squibb for 7 years.
Kevin received his Bachelor of Science in genetics from Cornell University and his doctorate in molecular biology from Princeton University/Columbia Medical School. He completed his post-doctoral fellowship in oncology at Harvard Medical School. Kevin also serves on the Scientific Advisory Board of Generation Bio and is an independent board Director for Ovid Therapeutics.
 Sandeep Menon, M.D., Ph.D. – Chief Development Officer
Sandeep Menon, M.D., Ph.D. – Chief Development Officer
Dr. Sandeep Menon joined Alnylam in 2023 with 18 years of industry experience. He oversees all clinical development and safety activities at Alnylam as Chief Development Officer.
Prior to joining Alnylam, Dr. Menon served as Senior Vice President of Early Clinical Development and Chief Scientific Officer of AI and Digital Sciences at Pfizer for almost 14 years. He was part of the R&D leadership and oversaw all clinical development of multiple assets in multiple therapeutic areas across Inflammation & Immunology, Internal Medicine, Oncology, Rare Disease and Anti-Infectives. He also led Pfizer’s Biomedicine AI Unit, a “dedicated” Drug Discovery and Translational AI Unit tasked with indication/molecule/population prioritization using cutting edge AI/ML methods. While at Pfizer, he played a critical leadership role in leading a Phase II success rate of 50% from 2016 to 2021; he also co-led the development of Paxlovid. Over the years, he has overseen the development of multiple assets including Xeljanz, Litfulo, CINBINQO, Xalkori, Ibrance, next generation CDK inhibitors, Gene Therapies and HYMPAVZI. He also established the PfIRe Lab (Pfizer Research and Innovation Lab), a highly instrumented clinical research lab using cutting-edge wearables and sensors to develop and validate novel, patient-centric clinical endpoints, enabling a better understanding of a patient’s disease state and response to therapy. Preceding this, Dr. Menon held various roles at Biogen and ICON overseeing clinical development of multiple assets and indications in multiple modalities.
Dr. Menon completed his medical degree at Karnataka University, India and worked as an Emergency Physician in Mumbai. He then completed his Master’s in Public Health (MPH) and Ph.D. in Biostatistics from Boston University. He later completed an MS in Translational Pharmacology from Ohio State University. He was a Research Fellow at Harvard Clinical Research Institute and serves as an adjunct faculty member at Boston University School of Public Health.
 Paul Nioi, Ph.D. – SVP, Research
Paul Nioi, Ph.D. – SVP, Research
Dr. Paul Nioi joined Alnylam in 2018 and is currently Senior Vice President of Research. He is responsible for Alnylam’s portfolio of preclinical siRNA assets across all disease areas. He oversees the discovery of new targets, identification of development candidates and the body of mechanistic preclinical work needed to advance programs into the clinic. Before joining Alnylam, Dr. Nioi worked at both Amgen and Schering-Plough in roles of increasing responsibility. He received his Ph.D. from the University of Dundee and BSc from the University of Edinburgh.
 Anna Borodovsky, Ph.D. – VP, Research
Anna Borodovsky, Ph.D. – VP, Research
Dr. Anna Borodovsky joined Alnylam in 2006 as a Scientist and has subsequently served in roles of increasing responsibility within Research. Anna was promoted to VP in February of 2023. Throughout her career at Alnylam Anna has focused on the advancement of GalNAc-conjugated siRNAs into the clinic and research support for clinical stage programs. Her work at Alnylam includes the discovery and pre-clinical development of inclisiran and cemdisiran. Most recently she has led the work on the pre-clinical development of novel siRNA targets in Alnylam’s liver targeting platform from target nomination to IND-enabling studies and progression into the clinic.
Anna received her Bachelor of Science in Chemistry from Emory University and a Ph.D. in Biological Chemistry and Molecular Pharmacology from Harvard University. She did a post-doctoral fellowship at Biogen and held a staff scientist position at the Broad Institute prior to joining Alnylam.
 Vasant Jadhav, Ph.D. – Chief Technology Officer
Vasant Jadhav, Ph.D. – Chief Technology Officer
Vasant Jadhav is Chief Technology Officer, SVP, at Alnylam Pharmaceuticals, where he leads the RNAi Platform Technology efforts including siRNA designs and delivery approaches. He has >20 years of experience in the field of oligonucleotide therapeutics focusing on delivery and mechanistic investigations. He joined Alnylam in early 2014 after 12 years at Sirna-Merck, where he focused on optimizing siRNA designs and conjugate-based delivery systems. He is co-inventor of multiple technologies including ESC+ siRNA design, REVERSIR, GEMINI (Bis-RNAi) and extra-hepatic delivery approaches. His contributions with the team on potency and specificity of siRNAs are utilized in multiple approved RNAi drugs and the deep pipeline at Alnylam. He has authored >30 peer-reviewed publications in prestigious journals such as Nature Biotechnology, Nature Communications, Nucleic Acids Research, three of which were recognized as ‘Paper of the Year’ in 2021, 2022 and 2024 by the Oligonucleotides Therapeutics Society.
Vasant obtained his Ph.D. in Biotechnology from the University of Pune in 1998 and completed a post-doctoral fellowship at University of Colorado, Boulder working on discovering ribozymes that support RNA world hypothesis.
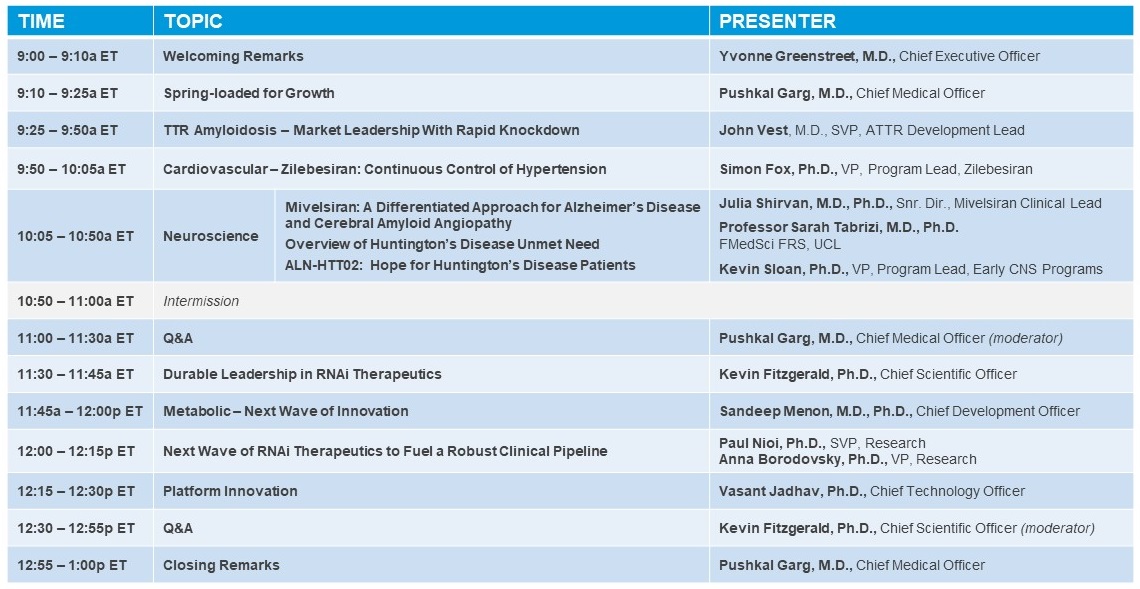
Agenda: