14 Dec, 2020 Alnylam R&D Day 2020
On December 15th and 16th, 2020, we hosted a virtual R&D Day event showcasing Alnylam’s late stage clinical efforts, next wave programs, and platform advances. The Company also discussed its perspective on its transition toward achieving a self-sustainable financial profile. The event included presentations from Alnylam management and key opinion leaders.
To view a replay of the webcast, click here.
To view the R&D Day presentation for Day 1, click here.
To view the R&D Day presentation for Day 2, click here.
Speakers – Alnylam Management:
 John Maraganore, Ph.D. – Chief Executive Officer
John Maraganore, Ph.D. – Chief Executive Officer
Since 2002, Dr. John Maraganore has served as the CEO and a Director of Alnylam.
Prior to Alnylam, Dr. Maraganore served as an officer and a member of the management team for Millennium Pharmaceuticals, Inc. As Senior Vice President, Strategic Product Development for Millennium, he was responsible for the company’s product franchises in oncology, and cardiovascular, inflammatory, and metabolic diseases. He was previously Vice President, Strategic Planning and M&A and, prior to that, he was General Manager of Millennium BioTherapeutics, Inc., a former subsidiary of Millennium. Before Millennium he served as Director of Molecular Biology and Director of Market and Business Development at Biogen, Inc. At Biogen, Dr. Maraganore invented and led the discovery and development of ANGIOMAX® (bivalirudin) for injection, formerly HIRULOGTM and currently marketed by The Medicines Company. Prior to Biogen, Dr. Maraganore was a scientist at ZymoGenetics, Inc., and the Upjohn Company.
Dr. Maraganore received his Master of Science and Ph.D. in biochemistry and molecular biology at the University of Chicago. He is a member of the Board for Agios Pharmaceuticals and Biotechnology Industry Organization (BIO) Board and is a member of the BIO Executive Committee.
 Yvonne Greenstreet, MBChB, MBA – President & Chief Operating Officer
Yvonne Greenstreet, MBChB, MBA – President & Chief Operating Officer
Dr. Greenstreet is President and Chief Operating Officer at Alnylam Pharmaceuticals, a company which has led the translation of RNA interference from Nobel Prize-winning discovery into an entirely new class of medicines. Yvonne has more than 25 years of experience in the Biopharmaceutical industry, driving strategy and innovation, bringing transformative medicines to patients and building successful businesses in the US, Europe and globally. Yvonne serves on the board of directors of Pacira Pharmaceuticals, American Funds, the Scientific Advisory Committee of the Bill and Melinda Gates Foundation and is a member of the Discovery Council of Harvard Medical School.
Between 2011 and 2013, Yvonne was Senior Vice President and Head of Medicines Development at Pfizer serving on the executive team leading a rapidly growing $16bn division. Prior to Pfizer, she was at GlaxoSmithKline plc for 18 years, where she was Senior Vice President and Chief of Strategy for Research and Development. Yvonne had previously been in various positions of increasing responsibility at GSK, including Senior Vice President for Medicines Development and Chief Medical Officer for Europe.
Yvonne trained as a physician and earned her medical degree from Leeds University in the UK and her MBA degree from INSEAD, France.
 Akshay Vaishnaw, M.D., Ph.D. – President, Research & Development
Akshay Vaishnaw, M.D., Ph.D. – President, Research & Development
Dr. Vaishnaw joined Alnylam Pharmaceuticals in 2006 as VP Clinical Research and has then subsequently served in various R&D roles with increasing responsibility. Akshay is currently the President of R&D. Prior to 2006, he was at Biogen as Senior Director, Translational Medicine.
Akshay received his M.D. from the University of Wales College of Medicine, U.K., and Ph.D. from the University of London, U.K., in Molecular Immunology. He is a Fellow of the Royal College of Physicians, U.K. Akshay is a member of the Board of Directors for Editas Medicine Inc. and Scholar Rock Inc. From 2014-2018, he served as a Board member of Visterra, Inc.
 John Vest, M.D. – Vice President, Clinical Research
John Vest, M.D. – Vice President, Clinical Research
Dr. John Vest joined Alnylam in 2015 and is currently Vice President of Clinical Research. He serves as the Global Clinical Lead for the ATTR Amyloidosis Franchise. In this role Dr. Vest oversees both the clinical development strategy and clinical trial execution for Alnylam’s innovative RNAi therapeutics targeting transthyretin. He has played an instrumental role in multiple pivotal Phase 3 studies of ONPATTRO and vutrisiran.
Before joining Alnylam, he worked in the Cardiovascular Translational Medicine group at Novartis where he played a key clinical role in developing the company’s early stage pipeline of compounds in the cardiovascular space. Prior to his career in industry, Dr. Vest spent over 16 years in the study and practice of medicine.
Dr. Vest received his MD from Columbia University where he went on to complete a residency in Internal Medicine and fellowship in Cardiovascular Disease. He subsequently completed sub-specialty training in Clinical Cardiac Electrophysiology at the Brigham and Women’s Hospital before returning to Columbia where he joined the faculty of the Cardiology Division.
 Lauren Melton – Senior Director, Program Leader, ALN-AGT
Lauren Melton – Senior Director, Program Leader, ALN-AGT
Lauren Melton initially joined Alnylam in 2012 and has over 15 years of Regulatory Affairs and drug development experience within the biotechnology industry. Lauren currently leads two drug development teams for Alnylam, overseeing the development of ALN-AGT, currently being investigated for the treatment of hypertension, and cemdisiran, currently being investigated for the treatment of IgA Nephropathy.
Prior to Alnylam, Lauren held Regulatory Affairs roles of increasing responsibility within the Rare Disease Unit at Genzyme and was the Global Regulatory Lead for Cerezyme® and Ceredelga®. Lauren also worked at Vertex Pharmaceuticals and was the Global Regulatory Lead for Orkambi®, and other development products.
Lauren received her B.S. in Biology from the University of Massachusetts, holds an M.S. from the Johns Hopkins University, and received an M.B.A. with Distinction, from the Johnson Graduate School of Management of Cornell University.
 Josh Friedman, M.D., Ph.D. – Senior Director, Clinical Research
Josh Friedman, M.D., Ph.D. – Senior Director, Clinical Research
Dr. Joshua Friedman has 7 years of clinical research and development experience. He joined Alnylam in 2019.
Prior to joining Alnylam, Dr. Friedman served as Director and Lead, Gastroenterology (GI) Translational Science at Janssen Research and Development. He oversaw all GI translational science and biomarker work from first in human through registration, including the approval of Stelara™ for ulcerative colitis.
Dr. Friedman received a Bachelor of Arts with high honors in Biochemistry from Harvard University and his M.D. and Ph.D. degrees from the University of Pennsylvania. He completed residency training in Pediatrics and fellowship training in Pediatric Gastroenterology at the Children’s Hospital of Philadelphia. He served on the faculty of the Perelman School of Medicine at the University of Pennsylvania and was appointed to Associate Professor prior to joining the industry.
 Pushkal Garg, M.D. – Chief Medical Officer
Pushkal Garg, M.D. – Chief Medical Officer
Dr. Pushkal Garg joined Alnylam in 2014 with 15 years of experience in clinical drug development. He oversees all clinical development activities at Alnylam.
Prior to joining Alnylam, Dr. Garg served as Vice President, Global Clinical Research, Immunoscience at Bristol-Myers Squibb (BMS). He was the strategic leader of the Immunoscience franchise and oversaw the development of multiple clinical assets across the areas of rheumatology, gastroenterology, nephrology, and transplantation. While at BMS, he was instrumental to the late-stage development and approval of Nulojix® (belatacept) for kidney transplant recipients, and for supplementary biologics license applications (BLAs) for Orencia® (abatacept). Preceding this, Dr. Garg held various roles at Millennium Pharmaceuticals, overseeing the clinical development of multiple small molecule and biologic therapeutics for the treatment of inflammatory disorders.
Dr. Garg received a Bachelor of Arts with high honors in Biochemistry from the University of California, Berkeley, and his M.D. from the University of California, San Francisco. He completed residency training in Internal Medicine at UCSF, was a fellow in the Robert Wood Johnson Clinical Scholars Program at Johns Hopkins University, and served on the faculty of Harvard Medical School and the Brigham & Women’s Hospital in Boston prior to joining the industry. Dr. Garg is a member of the Board of Directors of SQZ Biotechnologies (SQZ).
 Kevin Fitzgerald, Ph.D. – Senior Vice President & Chief Scientific Officer
Kevin Fitzgerald, Ph.D. – Senior Vice President & Chief Scientific Officer
Kevin Fitzgerald is Chief Scientific Officer, SVP, Head of Research at Alnylam Pharmaceuticals in Cambridge, MA. He has over 20 years of drug discovery experience. He joined Alnylam in 2005 as an Associate Director of research after spending 7 years at Bristol-Myers Squibb. He has served in roles of increasing responsibility and leadership since that time. His achievements at Alnylam include leadership of the company’s RNAi delivery efforts, resulting in two clinically validated modes of siRNA delivery, and the development of Alnylam’s RNAi therapeutic novel class of medicines. He is an inventor on over 50 patents including the majority of Alnylam’s marketed and pipeline programs, as well as an author of over 50 papers including many in prestigious journals such as Nature, Cell, and NEJM. He has led multiple programs — including Alnylam’s inclisiran program — from discovery through pre-clinical development, regulatory submissions, and early clinical development.
Kevin received his Bachelor of Science in genetics from Cornell University and his doctorate in molecular biology from Princeton University. He completed his post-doctoral fellowship in oncology at Harvard Medical School in oncology.
 Jeff Poulton – Chief Financial Officer
Jeff Poulton – Chief Financial Officer
Jeff joined Alnylam as CFO in August 2019. Prior to joining Alnylam, Jeff served as Chief Financial Officer at Indigo Agriculture, a plant microbiome company, from January 2018 to April 2019, where he supported the initial commercial scale-up of the business, including expansion outside the US.
Between 2003 and December 2017, Jeff held various roles of increasing responsibility at Shire Plc, culminating in his service as Chief Financial Officer and a member of Shire’s Executive Committee and Board of Directors from January 2015 to December 2017. During his tenure at Shire, Jeff also led Shire’s rare disease US, LATAM, and Asia Pacific commercial operations, as well as Shire’s global rare disease business unit. Prior to Shire, Jeff led corporate finance and business development initiatives in both the gas and electric utilities industry and the materials manufacturing sector, serving in financial leadership positions at Cinergy Corp and PPG Industries. Jeff also served in the United States Navy as a Commissioned Officer.
Jeff holds a BA in Economics from Duke University and an MBA in Finance from the Kelley School of Business at Indiana University. He is a member of the Board of Directors of EIP Pharmaceuticals and Homology Medicines.
Speakers – Key Opinion Leaders:
 Philip Hawkins, Ph.D., FRCP, FRCPath, FMedSci – National Amyloidosis Centre, Royal Free Hospital and University College London, UK
Philip Hawkins, Ph.D., FRCP, FRCPath, FMedSci – National Amyloidosis Centre, Royal Free Hospital and University College London, UK
Philip Hawkins is Founder and Professor of Medicine at the National Amyloidosis Centre, London, UK. He has worked in the field of systemic amyloidosis for 30 years, and his research has focused on the pathogenesis, diagnosis, monitoring and treatment of amyloidosis. He has developed new diagnostic imaging methods including SAP scintigraphy, DPD scintigraphy and cardiac MRI. He and his colleagues evaluate over 1000 new amyloidosis patients each year, and have contributed to the development of many new therapies.
 Akshay Desai, M.D., M.P.H – Director, Cardiomyopathy and Heart Failure Program, Cardiovascular Division, Brigham and Women’s Hospital; Associate Professor of Medicine, Harvard Medical School
Akshay Desai, M.D., M.P.H – Director, Cardiomyopathy and Heart Failure Program, Cardiovascular Division, Brigham and Women’s Hospital; Associate Professor of Medicine, Harvard Medical School
Akshay Desai, M.D., M.P.H. is the Director of the Cardiomyopathy and Heart Failure Program in the Advanced Heart Disease Section of the Cardiovascular Division, Brigham and Women’s Hospital and an Associate Professor of Medicine at Harvard Medical School (both in Boston, Massachusetts). He received his undergraduate education at Princeton University, where he graduated Summa Cum Laude in 1992 with an A.B. in Public and International Affairs. He was subsequently awarded a Rhodes Scholarship for study at Oxford University, where he completed an M. Phil. in European Politics and Society at Balliol College in 1994. Following on this, he began his medical training at Harvard Medical School where he was awarded the M.D. degree in 1998. He completed his internship and residency in Internal Medicine at Brigham and Women’s Hospital in 2001 and subsequently elected to pursue fellowship training in Cardiovascular Medicine at the same institution. During the final years of subspecialty training in cardiology, he completed additional fellowship training in Heart Failure and Transplantation under the direction of Dr. Lynne Stevenson. Concurrently, he conducted translational research in vascular medicine and diastolic heart failure under the supervision of Dr. Mark Creager. He was awarded an M.P.H. in 2004 from the Harvard School of Public Health. He currently is the Director of the Cardiomyopathy and Heart Failure Program in the Cardiovascular Division at Brigham and Women’s Hospital and divides his time between clinical care of patients with advanced heart disease and clinical research in cardiovascular clinical trials, emphasizing the pathophysiology, pharmacologic treatment, and ambulatory management of patients with heart failure.
 Arun J. Sanyal, MBBS, M.D. – Z. Reno Vlahcevic Professor of Medicine, Virginia Commonwealth University School of Medicine
Arun J. Sanyal, MBBS, M.D. – Z. Reno Vlahcevic Professor of Medicine, Virginia Commonwealth University School of Medicine
Arun J. Sanyal, M.D., is a Professor of Medicine, Physiology and Molecular Pathology in the Division of Gastroenterology at Virginia Commonwealth University (VCU) Medical Center in Richmond, Virginia. Dr. Sanyal also serves as Chairman of the NIH NASH Clinical Research Network, the NIMBLE consortium and the Liver Forum for NASH and fibrosis. His research interests include all aspects of NAFLD and NASH as well as complications of cirrhosis and end stage liver disease. He has been continuously funded by the NIH since 1995 and has over 350 peer reviewed publications with a H-index of 116. He was also recently ranked in top 0.01% of medical scientists based on their impact on the field (PlosBio 2019). He has served in several leadership roles nationally and globally including Chair of the Hepatobiliary Pathophysiology Study Section of the NIH, Secretary and then President of the American Association for Study of Liver Disease, founding member of the American Board of Internal Medicine Transplant Hepatology board examination and member of WHO advisory council on viral hepatitis. He is the co-founder and academic chair of the Liver Forum which is a platform to bring regulatory agencies (FDA and EMA) with industry and academics to accelerate drug development for NASH and advanced liver disease. He is currently engaged in numerous clinical trials and leads several phase 2B and 3 trials for NASH as well as complications of end stage liver disease. His contributions have been recognized by receiving the Distinguished Mentorship Award from the American Gastroenterological Association and the Distinguished Scientific Achievement Award from the American Liver Foundation in 2017 and the Distinguished Achievement Award from the AASLD in 2018.
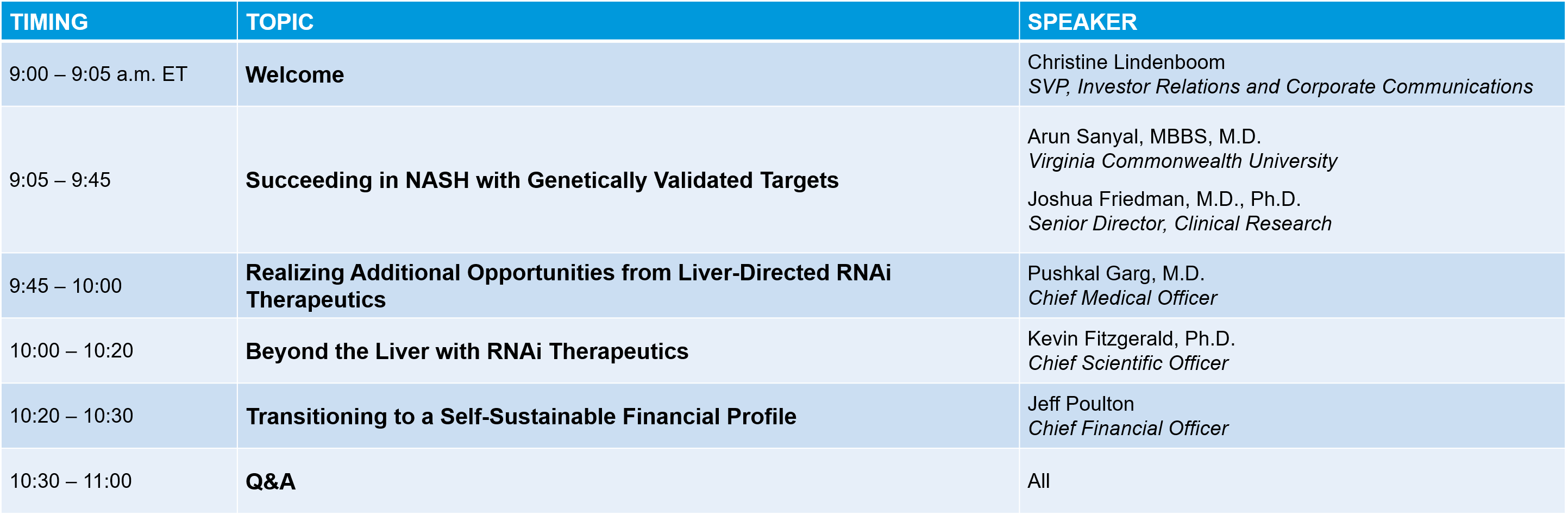
Agenda (Day 1)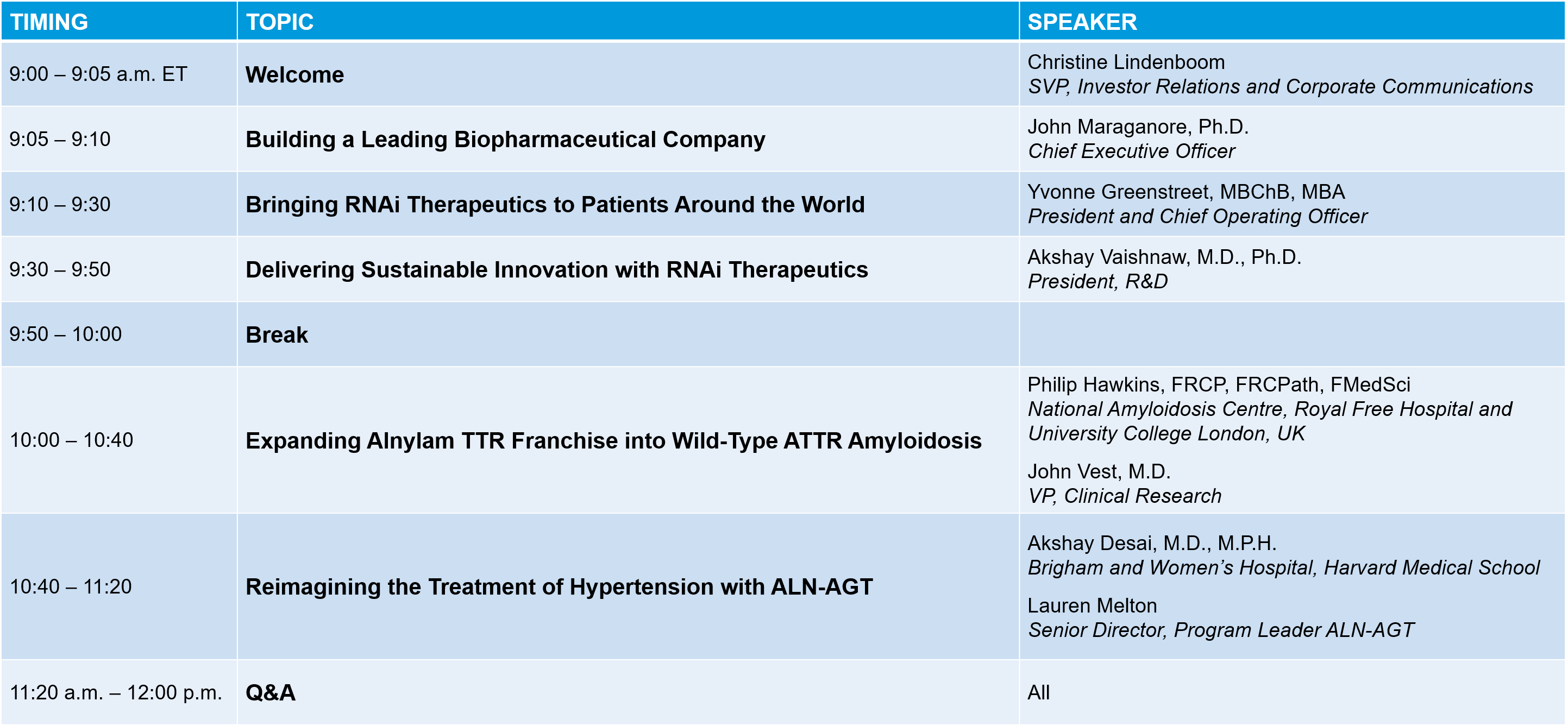
Agenda (Day 2)